The Test Includes the Following Assays Anti-C1q Anti-Phosphatidylserine Prothrombin PSPT IgM IgG. The AVISE Lupus test is composed of a two-tier multi-analyte algorithm to help facilitate evaluation of CTDs.
 Lupus Test Avise Ctd Test Report Sample
Lupus Test Avise Ctd Test Report Sample
EC4d has been shown to signiicantly correlate with disease activity20 FACS C3C4 IT Systemic Lupus.

Avise lupus test. Anti-C1q Anti-PhosphatidylserineProthrombin PSPT IgM IgG. AVISE Lupus is a 10-marker diagnostic test containing Cell-Bound Complement Activation Products CB-CAPs and SLE associated markers designed to aid healthcare providers in a timely differential diagnosis of SLE. THE BETTER COMPLEMENT TEST - Offering 22 greater sensitivity compared to low C3C4.
AVISE CTD has been validated to ofer 80 sensitivity 86 speciicity overal for BC4d SLE. AVISE SLE Monitor is composed of the following biomarkers Biomarkers associated with SLE disease activity Erythrocyte-bound C4d - EC4d. AVISE Lupus contains the following biomarkers.
The AVISE Lupus test is an ideal test for ANA positive patients with a clinical suspicion of lupus. AVISE CTD contains patented biomarkers and algorithms to provide improved diagnostic information compared to traditional lab tests alone. Given the AVISE Lupus tests improved performance 71 of suspected lupus cases are diagnosed within one year compared to 53 of suspected cases tested with SDLTs.
Background The AVISE Connective Tissue Disease CTD test uses autoantibody erythrocyte-bound C4d EC4d and B-cell-bound C4d BC4d levels to aid in diagnoses of SLE other CTDs and fibromyalgia. AVISE CTD is an advanced autoimmune rheumatic disease test specifically designed to aid physicians in the differential diagnosis of systemic lupus erythematosus SLE. The AVISE Lupus algorithmic two-tier index utilizes Exagens proprietary Cell-Bound Complement Activation Products CB-CAPs combined with SLE associated markers to provide a diagnostic tool with greater sensitivity over C3C4 and anti-dsDNA and greater specificity over antinuclear antibody ANA.
AVISE tests were developed and performance characteristics determined by Exagen Inc. AVISE CTD is a blood test that can help doctors diagnose lupus and other autoimmune diseases like rheumatoid arthritis Sjögrens syndrome or scleroderma. I believe I signed a document stating that I would not be charged more than 40 for the test and if my memory serves correctly thats exactly what I was charged.
I was negative on this one too. AVISE SLE Prognostic is a 10-marker panel developed to help assess a patients potential risk for Thrombosis Cardiovascular events Lupus Nephritis and Neuropsychiatric Lupus. Utility of the AVISE Connective Tissue Disease test in predicting lupus diagnosis and progression Lupus Sci Med.
This was the first test my rheumy ran after my doc and the hospital ran other negative sjogrens tests. Incorporated in AVISE CTD and AVISE Lupus to aid in diferential diagnosis of SLE compared to other common CTDs and primary Fibromyalgia. AVISE CTD is an advanced autoimmune rheumatic disease test powered by patented Cell-Bound Complement Activation Products CB-CAPs specifically designed to aid in the differential diagnosis of systemic lupus erythematosus SLE.
The AVISE CTD test can aid in SLE evaluation by predicting SLE disease development and future damage progression. By year two 87 of those with suspected lupus in the AVISE Lupus testing group are diagnosed with the disease compared to 75 in the SDLT group. The two new studies explored if the test provides useful information to doctors in real-world situations.
The test may be useful for people who have a positive antinuclear antibodies ANA test or anyone concerned they may have lupus or a similar autoimmune condition. Includes AVISE Lupus Profile BILLING INFORMATION. We evaluated the utility of the AVISE CTD test in predicting SLE disease development and damage progression.
AVISE Lupus developed by the company Exagen of Vista California also tests for complement molecules on blood cells and measures eight types of antibodies including antinuclear antibodies ANAs. AVISE SLE Prognostic is a 10-marker panel developed to help assess a patients potential risk for Thrombosis Cardiovascular events Lupus Nephritis and Neuropsychiatric Lupus. How AVISE CTD can help you.
The AVISE CTD test is an advanced blood test for lupus and connective tissue diseases. Insurance Patient Lab MEDICARE. The test includes the following assays.
AVISE SLE Monitor is an advanced SLE monitoring test comprised of six specialized biomarkers including patented EC4d and PC4d to help assess patients with SLE. AVISE CTD is the only diagnostic test powered by patented Cell-Bound Complement Activation Products CB-CAPs stable biomarkers of complement activation to help physicians better assess patients. While some components of AVISE tests are FDA approved devices the integrative test methods have not been cleared or approved by the FDA.
 Advanced Lupus Test Lupus Diagnosis Test Exagen Inc
Advanced Lupus Test Lupus Diagnosis Test Exagen Inc
 Lupus Prognosis Lupus Anticoagulant Test Avise Sle Prognostic
Lupus Prognosis Lupus Anticoagulant Test Avise Sle Prognostic
 Systemic Lupus Erythematosus Sle Test Exagen
Systemic Lupus Erythematosus Sle Test Exagen
 Avise Advanced Testing For Lupus And Autoimmune Disease
Avise Advanced Testing For Lupus And Autoimmune Disease
 Systemic Lupus Erythematosus Sle Test Exagen
Systemic Lupus Erythematosus Sle Test Exagen
 Lupus Test Avise Ctd Test Report Sample
Lupus Test Avise Ctd Test Report Sample
 The 2 Tiered Model Of The Avise Lupus Test Download Scientific Diagram
The 2 Tiered Model Of The Avise Lupus Test Download Scientific Diagram
The Lupus Encyclopedia Living With Lupus And Research Updates The Lupus Encyclopedia
 The Avise Lupus Test And Cell Bound Complement Activation Products Aid The Diagnosis Of Systemic Lupus Erythematosus Abstract Europe Pmc
The Avise Lupus Test And Cell Bound Complement Activation Products Aid The Diagnosis Of Systemic Lupus Erythematosus Abstract Europe Pmc
 Advanced Lupus Test Lupus Diagnosis Test Exagen Inc
Advanced Lupus Test Lupus Diagnosis Test Exagen Inc
Https Www Exagen Com Wp Content Uploads Interpretation Guide Combined Sa1123 E 2 Pdf
 Systemic Lupus Erythematosus Sle Test Exagen
Systemic Lupus Erythematosus Sle Test Exagen
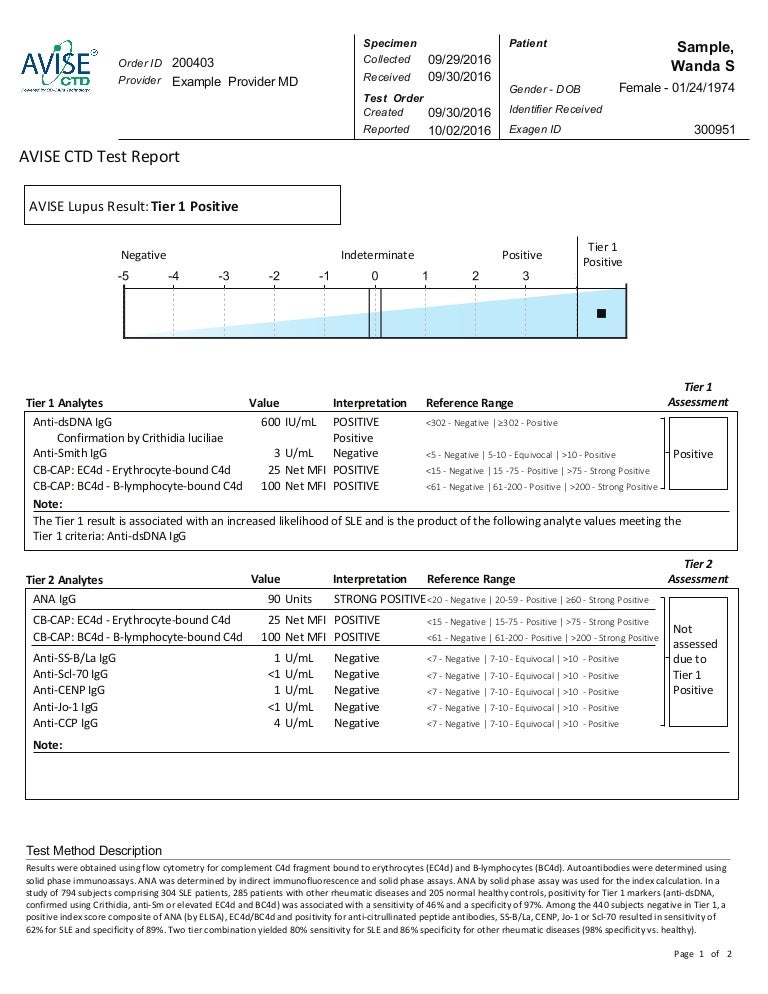 Lupus Test Avise Ctd Test Report Sample
Lupus Test Avise Ctd Test Report Sample
 Lupus Diagnosis And Management 3 Tests That May Be Right For You Lupus Chick
Lupus Diagnosis And Management 3 Tests That May Be Right For You Lupus Chick
No comments:
Post a Comment
Note: Only a member of this blog may post a comment.