The bioavailability of subcutaneous azacitidine is approximately 89 compared with IV azacitidine based on AUC. VIDAZA azacitidine for injection for SC or IV use Initial US.
 Azacitidine Taj Pharma 100 Mg Ml Powder For Injection Taj Generics Pharmaceuticals Taj Pharma
Azacitidine Taj Pharma 100 Mg Ml Powder For Injection Taj Generics Pharmaceuticals Taj Pharma
Azacitidine is rapidly absorbed after subcutaneous administration.
Azacitidine package insert. Medically reviewed by Judith Stewart BPharm. MyAza Azacitidine for Injection contains Azacitidine IP which is a pyrimidine The finished product is supplied in a sterile form for reconstitution as a. - SubQ or intravenous IV infusion over 10 to 40 minutes on Days 1 2 3 4 5 6 and 7 Estimated total infusion time for this treatment.
Available generically through various manufacturers VII. 2 OSHA Technical Manual TED 1-015A Section VI. AZACITIDINE PACKAGE INSERT PDF.
Refractory anemia RA or refractory anemia with ringed sideroblasts if accompanied by neutropenia or thrombocytopenia or requiring transfusions refractory anemia with excess blasts RAEB refractory anemia. January 9 2020 admin. AZACITIDINE PACKAGE INSERT PDF.
PACKAGE INSERT DATA. 2004 -----INDICATIONS AND USAGE----- VIDAZA is a nucleoside metabolic inhibitor indicated for the treatment of patients with the following FAB myelodysplastic syndrome MDS subtypes. Inject the diluent slowly into the vial.
Vigorously shake or roll the vial until a uniform suspension is achieved. The resulting suspension will contain azacitidine 25 mgmL. Azacitidine for injection is indicated for treatment of patients with the following French-American-British FAB myelodysplastic syndrome subtypes.
Preparation for Immediate Subcutaneous Administration. No formal clinical drug interaction studies with azacitidine have been conducted. The suspension will be cloudy.
Azacitidine ay za SYE ti deen Brand Name. Doing so could remove the active substance. Refractory anemia RA or refractory anemia with ringed sideroblasts if accompanied by neutropenia or thrombocytopenia or requiring transfusions refractory anemia with excess blasts RAEB refractory anemia with.
The product may be held at room temperature for up to. Vigorously shake or roll the vial until a uniform suspension is. ONUREG azacitidine is indicated for continued treatment of adult patients with acute myeloid leukemia who achieved first complete remission CR or complete remission with incomplete blood count recovery CRi following intensive induction chemotherapy and are.
VIDAZA azacitidine injection Package insert. Azacitidine for injection is indicated for treatment of patients with the following French-American-British FAB myelodysplastic syndrome subtypes. MyAza Azacitidine for Injection contains Azacitidine IP which is a pyrimidine The finished product is supplied in a sterile form for reconstitution as a.
No formal clinical drug interaction studies with azacitidine. Azacitidine subcutaneous injection SQ. Up to one hour for each treatment.
Do not filter the suspension after reconstitution. Reconstitute Azacitidine for Injection aseptically with 4 mL sterile water for injection. 1 billable unit 1mg NDC.
Azacitidine package insert pdf October 16 2019 admin Science MyAza Azacitidine for Injection contains Azacitidine IP which is a pyrimidine The finished product is supplied in a sterile form for reconstitution as a. SubQ may only take a few minutes but add about 30 minutes or longer wait time for drug preparation. Doses greater than 4 mL should be divided equally into 2 syringes.
No formal clinical drug interaction studies with azacitidine. J9025 Injection azacitidine 1 mg. Onureg azacitidine is a prescription medicine used for continued treatment of adults with acute myeloid leukemia.
1 Preventing Occupational Exposures to Antineoplastic and Other Hazardous Drugs in Health Care Settings. Inject the diluent slowly into the vial. Do not filter the suspension after reconstitution.
VIDAZA azacitidine for injection for subcutaneous or intravenous use Initial US. Last updated on Nov 16 2020. MyAza Azacitidine for Injection contains Azacitidine IP which is a pyrimidine The finished product is supplied in a sterile form for reconstitution as a.
Reconstitute VIDAZA aseptically with 4 mL sterile water for injection. In 21 patients with cancer subcutaneous azacitidine administration resulted in AUC and Cmax values that were dose proportional over a dose range of 25 mgm2 to 100. AZACITIDINE injection powder lyophilized for solution.
AZACITIDINE PACKAGE INSERT PDF. 2004 -----INDICATIONS AND USAGE----- VIDAZA is a nucleoside metabolic inhibitor indicated for the treatment of patients with the following FAB myelodysplastic syndrome MDS subtypes. Vidaza 100mg powder injection.
 Vidaza Fda Prescribing Information Side Effects And Uses
Vidaza Fda Prescribing Information Side Effects And Uses
Fresenius Kabi Usa Product Details
Https Www Ema Europa Eu Documents Product Information Azacitidine Mylan Epar Product Information En Pdf
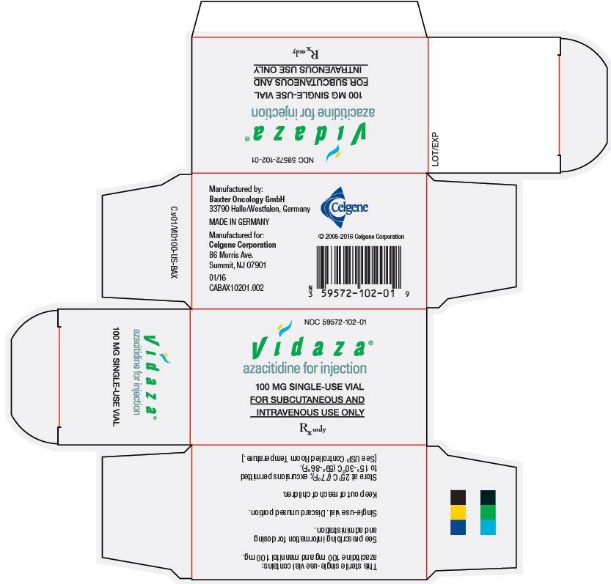 Ndc 59572 102 Vidaza Azacitidine
Ndc 59572 102 Vidaza Azacitidine
 Oral Aml Therapy Onureg Gets Fda Approval Mpr
Oral Aml Therapy Onureg Gets Fda Approval Mpr
 Samyang Biopharmaceuticals Corporation Successfully Launched Its Anti Cancer Treatment Drug Azacitidine Injection In The Eu For The First Time As A Korean Companynews Information Center Samyang Holdings Biopharmaceuticals Division
Samyang Biopharmaceuticals Corporation Successfully Launched Its Anti Cancer Treatment Drug Azacitidine Injection In The Eu For The First Time As A Korean Companynews Information Center Samyang Holdings Biopharmaceuticals Division
 Ndc 59572 102 Vidaza Azacitidine
Ndc 59572 102 Vidaza Azacitidine
 Azacitidine Bluepoint Laboratories Fda Package Insert Page 5
Azacitidine Bluepoint Laboratories Fda Package Insert Page 5
 Azacitidine Injection Fda Prescribing Information Side Effects And Uses
Azacitidine Injection Fda Prescribing Information Side Effects And Uses
 Pdf Fda Drug Approval Summary Azacitidine 5 Azacytidine Vidazatm For Injectable Suspension
Pdf Fda Drug Approval Summary Azacitidine 5 Azacytidine Vidazatm For Injectable Suspension
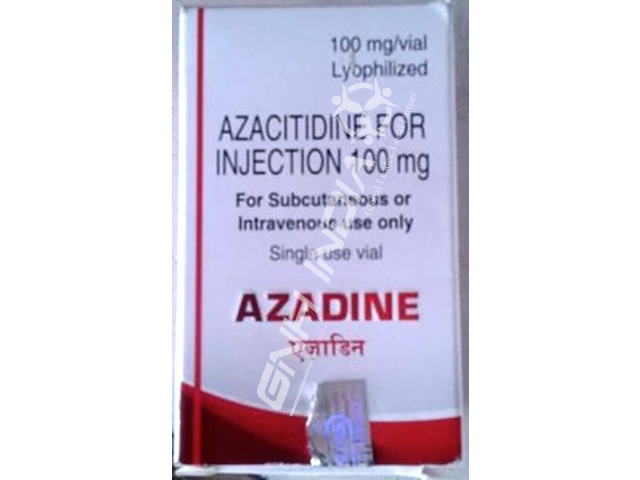 Buy Azacitidine 100mg Azadine No No
Buy Azacitidine 100mg Azadine No No
 Azacitidine Accord Healthcare Inc Fda Package Insert
Azacitidine Accord Healthcare Inc Fda Package Insert
Http E Lactancia Org Media Papers Azacitidine Ds Celgene2018 Pdf
 Azacitidine Taj Pharma 100 Mg Ml Powder For Injection Taj Generics Pharmaceuticals Taj Pharma
Azacitidine Taj Pharma 100 Mg Ml Powder For Injection Taj Generics Pharmaceuticals Taj Pharma
No comments:
Post a Comment
Note: Only a member of this blog may post a comment.